
The behaviour of plasma is now well-understood, and so the building of a power reactor is simply a matter of overcoming engineering hurdles – work is expected to commence on the DEMO fusion reactor in 2030. Nuclear fission is the splitting of atomic nuclei, while nuclear fusion is the combination of atomic nuclei true or false How are isotopes of an element the. The temperature has been achieved (over 100 million degrees), however confining the hot fuel plasma using powerful magnetic fields has taken a while to perfect. There is no chain reaction involved – hence there can not be an explosion – the reaction is achieved simply by getting the fuel hot enough and containing it tightly enough for the components to collide and fuse. Both fission and fusion reactions result in change of elements reacting. The other main difference between fusion and fission is that fission can be made to occur relatively easily, by assembling a sufficient mass of uranium or. This chain reaction is the key to fission reactions, but it can lead to a runaway process, as in a nuclear bomb.įusion is a much harder reaction to achieve, however it yields more energy than fission. Hint Fission reactions power the Sun, while the fusion reactions keep Earths core warm. The result of the instability is the nucleus breaking up (in any one of many different ways), in the process producing more neutrons, which in turn hit more uranium atoms and make them unstable and so on.
As stated previously, a radioisotope is defined as a nucleus that contains too many neutrons and, therefore, is highly unstable. Classify a nuclear reaction as a fission reaction or a fusion reaction.
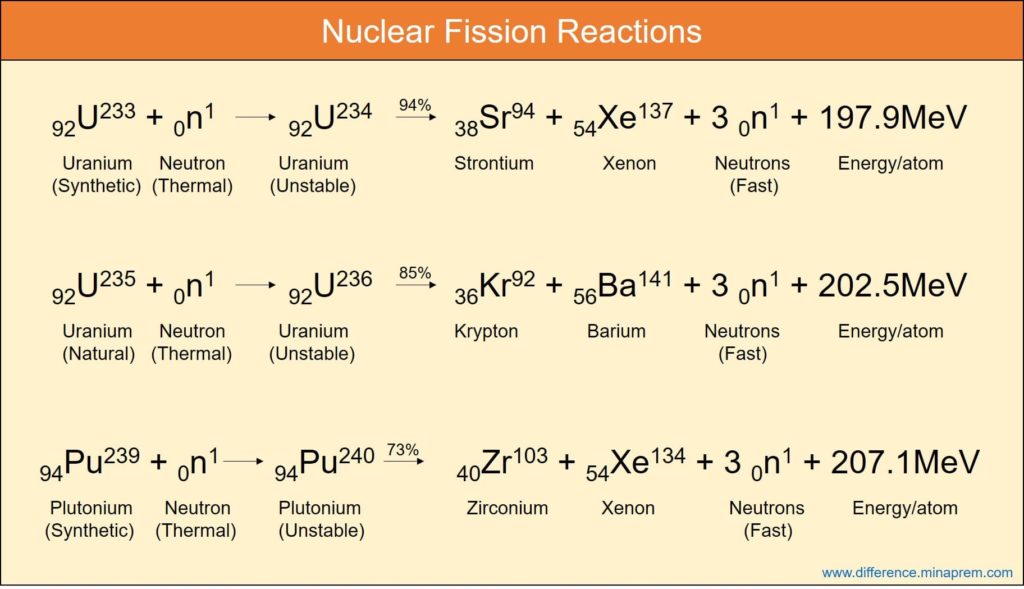
Both reactions release energy which, in a power plant, would be used to boil water to drive a steam generator, thus producing electricity.įission is triggered by uranium absorbing a neutron, which renders the nucleus unstable. Learning Objectives Define fission reaction. In fission, energy is gained by splitting apart heavy atoms (uranium) into smaller atoms (such as iodine, caesium, strontium, xenon and barium, to name just a few) whereas fusion is combining light atoms (in current experiments two isotopes of hydrogen, deuterium and tritium), which form a heavier one (helium). The energy harnessed in nuclei is released in nuclear reactions. Results: The main result is that fission reactions are always spontaneous (G < 0) since a lot of energy is released in the form of heat and the.


 0 kommentar(er)
0 kommentar(er)
